
Chapter Three: Cell Biology
Cell Membrane - Structure
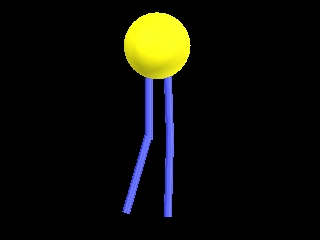 |
This is a simple representation of a phospholipid. the yellow structure represents
the hydrophillic or water loving section of the phospholipid. The blue tails that
come off of the sphere represent the hydrophobic or water fearing end of the
Phospholipid. Below is a structural model of a phospholipid that explains what these
terms mean.
|
|
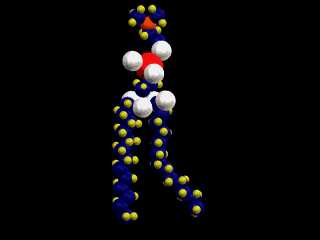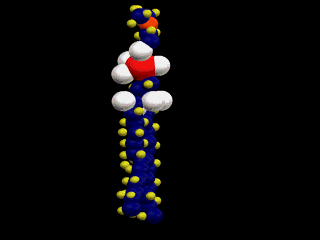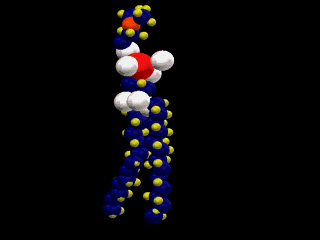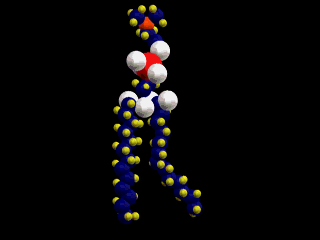
The two long chains coming off of the bottom of this molecule are made up of carbon
and hydrogen. Because both of these elements share their electrons evenly these
chains have no charge (gasoline is also a hydrocarbon). Molecules with no charge
are not attracted to water; as a result water molecules tend to push them out of
the way as they are attracted to each other. This causes molecules with no charge
not to dissolve in water (this is why gasoline and water do not mix).
At the other end of the phospholipid is a phosphate group and several double
bonded oxygens. The atoms at this end of the molecule are not shared equally. This
end of the molecule has a charge and is attracted to water.
|
 |
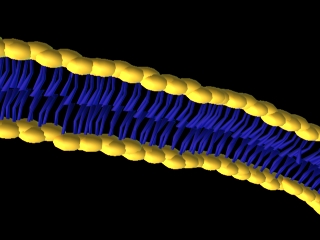 |
If you mix phospholipids in water they will form these double layered structures.
The hydrophillic ends will be in contact with water. The hydrophibic ends will face
inwards touching each other.
|




